Abstract
Background: Enasidenib (AG-221) is an oral, selective inhibitor of mIDH2 proteins. Results from the AG221-C-001 phase 1/2 dose-escalation and expansion study of enasidenib monotherapy showed an overall response rate (ORR) of 40.3% and median overall survival (OS) of 9.3 months in patients with m IDH2 relapsed or refractory (R/R) AML (Stein, Blood, 2017). Like patients with R/R AML, older patients with untreated AML who are not candidates for standard induction therapy due to advanced age, poor performance status, comorbidities, poor-risk cytogenetics, or other factors, pose a therapeutic challenge. Treatment options for these patients are limited and outcomes are poor. Reported here are clinical outcomes for older patients with previously untreated m IDH 2 AML who received enasidenib monotherapy in the AG221-C-001 study (NCT01915498).
Methods: The phase 1 dose-escalation and expansion portions of the study included patients aged ≥ 60 years with previously untreated AML who were not candidates for standard treatment and had ECOG PS scores of 0-2. Patients in the dose-escalation phase received enasidenib doses of 50-650 mg/day, and all patients in the expansion phase received enasidenib 100 mg/day, in continuous 28-day treatment cycles. ORR included complete remission (CR), CR with incomplete count recovery (CRi/CRp), partial remission (PR), and morphologic leukemia-free state (MLFS), per modified IWG 2003 response criteria for AML. OS was defined as the time from first dose to death from any cause. Event-free survival (EFS) was defined as the time from first dose to relapse, progressive disease (PD), or death, whichever came first. Safety was assessed by treatment-emergent adverse event (TEAE) reporting and TEAEs were graded for severity per CTCAE version 4.0.
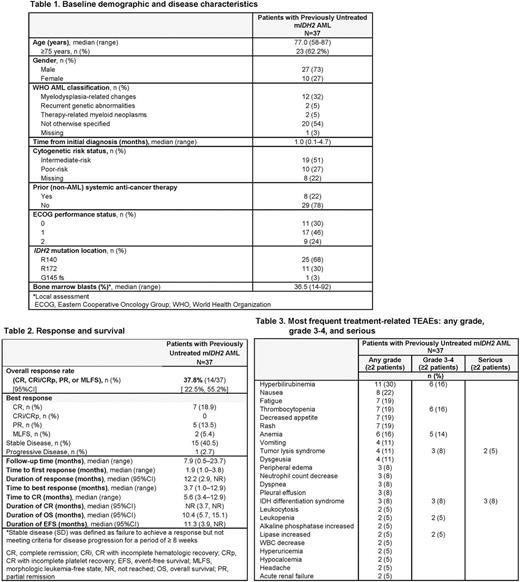
Results: Of 239 patients in the phase 1 dose-escalation and study expansion, 37 patients (15.5%) had previously untreated m IDH2 AML. At data cutoff (14 Oct 2016), 4 patients with previously untreated AML (11%) remained on-study: 3 patients in CR, and 1 patient with stable disease at cycle 13. Median age was 77 years (range 58-87); 62% of patients were aged ≥ 75 years (Table 1). Median number of enasidenib treatment cycles was 6 (range 1-23) and median follow-up was 7.9 months (range 0.5-23.7). Seven patients (19%) attained CR, with a median time to CR of 5.6 months (range 3.4-12.9) (Table 2). ORR was 37.8% (95%CI 22.5, 55.2). The median duration of CR was not reached (NR) (95%CI 3.7, NR) and median duration of any response was 12.2 months (2.9, NR) (Table 2). Three patients proceeded to transplant; at data cutoff, all 3 patients remained in remission. Among all 37 patients, median OS was 10.4 months (95%CI 5.7, 15.1) and median EFS was 11.3 months (3.9, NR). Median OS for responding patients (n=14) was 19.8 months (95%CI 10.4, NR) and for non-responders was 5.4 months (2.8, 12.4). The most frequent TEAEs (any grade or cause) were fatigue (43%), nausea (41%), and decreased appetite (41%). The most frequent treatment-related TEAEs were hyperbilirubinemia (30%) and nausea (22%) (Table 3). The only serious treatment-related TEAEs reported for more than 1 patient were IDH differentiation syndrome (n=3, 8%) and tumor lysis syndrome (n=2, 5%). Treatment-related TEAEs led to dose modification for 3 patients (8%), dose interruption for 7 patients (19%), and treatment discontinuation for 1 patient (3%).
Conclusions: Enasidenib induced hematologic responses in these older patients with previously untreated m IDH2 AML who were not candidates for standard treatment. Approximately 1 in 5 of these patients attained CR and 1 in 3 patients had a response with enasidenib monotherapy. Responses were durable: at a median of 7.9 months of follow-up, median CR duration was not reached and median duration of any response was > 1 year. Median OS and EFS were also promising (10.4 months and 11.3 months, respectively). Rates of treatment-related TEAEs were low and only 1 patient discontinued treatment due to a TEAE. These results suggest enasidenib may benefit older adults with m IDH2 AML who are not fit to receive cytotoxic chemotherapy. These encouraging findings have prompted follow-up studies of enasidenib in older patients with previously untreated m IDH2 AML, such as the Beat AML Master Trial (NCT03013998).
Pollyea: Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis: Membership on an entity's Board of Directors or advisory committees; Agios, Pfizer: Research Funding. De Botton: Servier: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Agios: Honoraria, Research Funding. DiNardo: Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding. Kantarjian: Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Novartis: Research Funding; ARIAD: Research Funding; Pfizer: Research Funding; Delta-Fly Pharma: Research Funding. Collins: BMS: Research Funding; Arog: Research Funding; Agios: Research Funding; Celgene Corporation: Research Funding. Stein: Amgen: Consultancy, Speakers Bureau; Stemline: Consultancy. Xu: Celgene Corporation: Employment, Equity Ownership. Tosolini: Celgene Corporation: Employment, Equity Ownership. Gupta: Celgene Corporation: Employment, Equity Ownership. Agresta: Agios Pharmaceuticals, Inc.: Employment, Equity Ownership. Stein: Seattle Genetics: Research Funding; GSK: Other: Advisory Board, Research Funding; Constellation Pharma: Research Funding; Celgene Corporation: Consultancy, Other: Travel expenses, Research Funding; Agios Pharmaceuticals, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Other: Travel expenses; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal